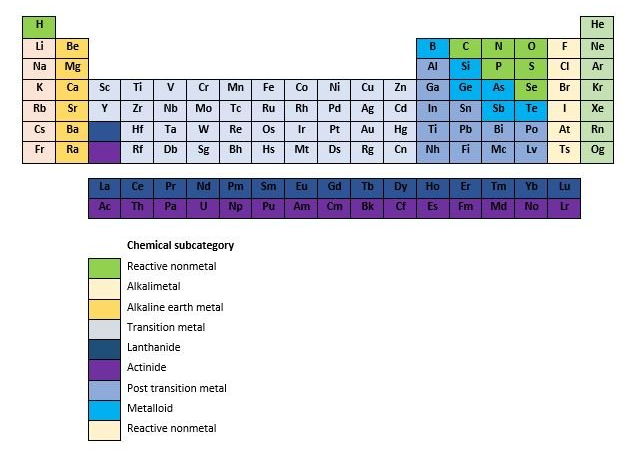
Periodic table of elements
The chemical elements of the periodic table alphabetically listed
- Ac Actinium 89
Symbol Ac
Discovery and first isolation Friedrich Oskar Giesel (1902)
Named by André-Louis Debierne (1899)
Appearance silvery-white, glowing with an eerie blue light; sometimes with a golden cast
Mass number [227]
Atomic number (Z) 89
Group group 3
Period period 7
Block d-block
Element category actinide, sometimes considered a transition metal
Electron configuration [Rn] 6d1 7s2
Electrons per shell 2, 8, 18, 32, 18, 9, 2
- Ag Silver 47
Symbol Ag
Discovery before 5000 BC
Appearance lustrous white metal
Standard atomic weight Ar, std(Ag) 107.8682(2)
Atomic number (Z) 47
Group group 11
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d10 5s1
Electrons per shell
2, 8, 18, 18, 1
- Al Aluminium 13
Atomic number (Z) 13
Naming after alumina (aluminium oxide), itself named after mineral alum
Prediction Antoine Lavoisier (1782)
Discovery and first isolation Hans Christian Ørsted (1824)
Named by Humphry Davy (1812)
Appearance silvery gray metallic
Standard atomic weight Ar, std(Al) 26.9815384(3)
Group group 13 (boron group)
Period period 3
Block p-block
Element category Post-transition metal, [2][a] sometimes considered a metalloid
Electron configuration [Ne] 3s2 3p1
Electrons per shell 2, 8, 3
- Am Americium 95
Symbol Am
Naming after the Americas
Discovery Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, Albert Ghiorso (1944)
Appearance silvery white
Mass number [243]
Atomic number (Z) 95
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f7 7s2
Electrons per shell
2, 8, 18, 32, 25, 8, 2
- Ar Argon 18
Symbol Ar
Discovery and first isolation Lord Rayleigh and William Ramsay (1894)
Atomic number (Z) 18
Appearance colorless gas exhibiting a lilac/violet glow when placed in an electric field
Standard atomic weight Ar, std(Ar) [39.792, 39.963] conventional: 39.95
Group group 18 (noble gases)
Period period 3
Block p-block
Element category noble gas
Electron configuration [Ne] 3s2 3p6
Electrons per shell
2, 8, 8
- As Arsenic 33
Symbol As
Discovery Arabic alchemists (before AD 815)
Appearance metallic grey
Standard atomic weight Ar, std(As) 74.921595(6)
Atomic number (Z) 33
Group group 15 (pnictogens)
Period period 4
Block p-block
Element category metalloid
Electron configuration [Ar] 3d10 4s2 4p3
Electrons per shell
2, 8, 18, 5
- At Astatine 85
Symbol At
Naming after Greek astatos (αστατος), meaning "unstable"
Discovery Dale R. Corson, Kenneth Ross MacKenzie, Emilio Segrè (1940)
Appearance unknown, probably metallic
Mass number [210]
Atomic number (Z) 85
Group group 17 (halogens)
Period period 6
Block p-block
Element category metalloid, sometimes classified as a nonmetal, or a metal[1][2]
Electron configuration [Xe] 4f14 5d10 6s2 6p5
Electrons per shell
2, 8, 18, 32, 18, 7
- Au Gold 79
Symbol Au
Naming from Latin aurum, meaning gold
Discovery In the Middle East (before 6000 BCE)
Appearance metallic yellow
Standard atomic weight Ar, std(Au) 196.966570(4)
Atomic number (Z) 79
Group group 11
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d10 6s1
Electrons per shell
2, 8, 18, 32, 18, 1
- B Boron 5
Symbol B
Discovery Joseph Louis Gay-Lussac and Louis Jacques Thénard (30 June 1808)
First isolation Humphry Davy (9 July 1808)
Appearance black-brown
Standard atomic weight Ar, std(B) [10.806, 10.821] conventional: 10.81
Atomic number (Z) 5
Group group 13 (boron group)
Period period 2
Block p-block
Element category metalloid
Electron configuration [He] 2s2 2p1
Electrons per shell
2, 3
- Ba Barium 56
Symbol Ba
Discovery Carl Wilhelm Scheele (1772)
First isolation Humphry Davy (1808)
Appearance silvery gray; with a pale yellow tint
Standard atomic weight Ar, std(Ba) 137.327(7)
Atomic number (Z) 56
Group group 2 (alkaline earth metals)
Period period 6
Block s-block
Element category alkaline earth metals
Electron configuration [Xe] 6s2
Electrons per shell
2, 8, 18, 18, 8, 2
- Be Beryllium 4
Symbol Be
Discovery Louis Nicolas Vauquelin (1798)
First isolation Friedrich Wöhler & Antoine Bussy (1828)
Appearance white-gray metallic
Standard atomic weight Ar, std(Be) 9.0121831(5)
Atomic number (Z) 4
Group group 2 (alkaline earth metals)
Period period 2
Block s-block
Element category alkaline earth metal
Electron configuration [He] 2s2
Electrons per shell
2, 2
- Bh Bohrium 107
Symbol Bh
Naming after Niels Bohr
Discovery Gesellschaft für Schwerionenforschung (1981)
Mass number [270] (unconfirmed: 278)
Atomic number (Z) 107
Group group 7
Period period 7
Block d-block
Element category transition metal
Electron configuration [Rn] 5f14 6d5 7s2[1][2]
Electrons per shell
2, 8, 18, 32, 32, 13, 2
- Bi Bismuth 83
Symbol Bi
Discovery Arabic alchemists (before AD 1000)
Appearance lustrous brownish silver
Standard atomic weight Ar, std(Bi) 208.98040(1)
Atomic number (Z) 83
Group group 15 (pnictogens)
Period period 6
Block p-block
Element category post-transition metal
Electron configuration [Xe] 4f14 5d10 6s2 6p3
Electrons per shell
2, 8, 18, 32, 18, 5
- Bk Berkelium 97
Symbol Bk
Naming after Berkeley, California, where it was discovered
Discovery Lawrence Berkeley National Laboratory (1949)
Appearance silvery
Mass number [247]
Atomic number (Z) 97
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f9 7s2
Electrons per shell
2, 8, 18, 32, 27, 8, 2
- Br Bromine 35
Symbol Br
Discovery and first isolation Antoine Jérôme Balard and Carl Jacob Löwig (1825)
Appearance reddish-brown
Standard atomic weight Ar, std(Br) [79.901, 79.907] conventional: 79.904
Atomic number (Z) 35
Group group 17 (halogens)
Period period 4
Block p-block
Element category reactive nonmetal
Electron configuration [Ar] 3d10 4s2 4p5
Electrons per shell
2, 8, 18, 7
- C Carbon 6
Symbol C
Discovery Egyptians and Sumerians (3750 BCE)
Recognized as an element by Antoine Lavoisier (1789)
Appearance
graphite: black
diamond: clear
Standard atomic weight Ar, std(C) [12.0096, 12.0116] conventional: 12.011
Atomic number (Z) 6
Group group 14 (carbon group)
Period period 2
Block p-block
Element category reactive nonmetal, sometimes considered a metalloid
Electron configuration [He] 2s2 2p2
Electrons per shell
2, 4
- Ca Calcium 20
Symbol Ca
Discovery and first isolation Humphry Davy (1808)
Appearance dull gray, silver; with a pale yellow tint
Standard atomic weight Ar, std(Ca) 40.078(4)
Atomic number (Z) 20
Group group 2 (alkaline earth metals)
Period period 4
Block s-block
Element category alkaline earth metal
Electron configuration [Ar] 4s2
Electrons per shell
2, 8, 8, 2
- Cd Cadmium 48
Symbol Cd
Discovery and first isolation Karl Samuel Leberecht Hermann and Friedrich Stromeyer (1817)
Named by Friedrich Stromeyer (1817)
Appearance silvery bluish-gray metallic
Standard atomic weight Ar, std(Cd) 112.414(4)
Atomic number (Z) 48
Group group 12
Period period 5
Block d-block
Element category post-transition metal, alternatively considered a transition metal
Electron configuration [Kr] 4d10 5s2
Electrons per shell
2, 8, 18, 18, 2
- Ce Cerium 58
Symbol Ce
Naming after dwarf planet Ceres, itself named after Roman deity of agriculture Ceres
Discovery Martin Heinrich Klaproth, Jöns Jakob Berzelius, Wilhelm Hisinger (1803)
First isolation Carl Gustaf Mosander (1838)
Appearance silvery white
Standard atomic weight Ar, std(Ce) 140.116(1)
Atomic number (Z) 58
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f1 5d1 6s2[2]
Electrons per shell
2, 8, 18, 19, 9, 2
- Cf Californium 98
Symbol Cf
Naming after California, where it was discovered
Discovery Lawrence Berkeley National Laboratory (1950)
Appearance silvery
Mass number [251]
Atomic number (Z) 98
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f10 7s2[1]
Electrons per shell
2, 8, 18, 32, 28, 8, 2
- Cl Chlorine 17
Symbol Cl
Discovery and first isolation Carl Wilhelm Scheele (1774)
Recognized as an element by Humphry Davy (1808)
Appearance pale yellow-green gas
Standard atomic weight Ar, std(Cl) [35.446, 35.457] conventional: 35.45
Atomic number (Z) 17
Group group 17 (halogens)
Period period 3
Block p-block
Element category reactive nonmetal
Electron configuration [Ne] 3s2 3p5
Electrons per shell
2, 8, 7
- Cm Curium 96
Symbol Cm
Naming named after Marie Skłodowska-Curie and Pierre Curie
Discovery Glenn T. Seaborg, Ralph A. James, Albert Ghiorso (1944)
Appearance silvery metallic, glows purple in the dark
Mass number [247]
Atomic number (Z) 96
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f7 6d1 7s2
Electrons per shell
2, 8, 18, 32, 25, 9, 2
- Co Cobalt 27
Symbol Co
Discovery and first isolation Georg Brandt (1735)
Appearance hard lustrous bluish gray metal
Standard atomic weight Ar, std(Co) 58.933194(3)
Atomic number (Z) 27
Group group 9
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d7 4s2
Electrons per shell
2, 8, 15, 2
- Cr Chromium 24
Symbol Cr
Discovery and first isolation Louis Nicolas Vauquelin (1794, 1797)
Appearance silvery metallic
Standard atomic weight Ar, std(Cr) 51.9961(6)
Atomic number (Z) 24
Group group 6
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d5 4s1
Electrons per shell
2, 8, 13, 1
- Cs Cesium/Caesium 55
Symbol Cs
Naming from Latin caesius, sky blue, for its spectral colours
Discovery Robert Bunsen and Gustav Kirchhoff (1860)
First isolation Carl Setterberg (1882)
Appearance pale gold
Standard atomic weight Ar, std(Cs) 132.90545196(6)
Atomic number (Z) 55
Group group 1 (alkali metals)
Period period 6
Block s-block
Element category alkali metal
Electron configuration [Xe] 6s1
Electrons per shell
2, 8, 18, 18, 8, 1
- Cu Copper 29
Symbol Cu
Naming after Cyprus, principal mining place in Roman era (Cyprium)
Discovery Middle East (9000 BC)
Appearance red-orange metallic luster
Standard atomic weight Ar, std(Cu) 63.546(3)
Atomic number (Z) 29
Group group 11
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d10 4s1
Electrons per shell
2, 8, 18, 1
- Cn Copernicium 112
Symbol Cn
Naming after Nicolaus Copernicus
Discovery Gesellschaft für Schwerionenforschung (1996)
Mass number [285]
Atomic number (Z) 112
Group group 12
Period period 7
Block d-block
Element category post-transition metal, alternatively considered a transition metal
Electron configuration [Rn] 5f14 6d10 7s2 (predicted)[1]
Electrons per shell
2, 8, 18, 32, 32, 18, 2 (predicted)
- Db Dubnium 105
Symbol Db
Naming after Dubna, Moscow Oblast, Russia, site of Joint Institute for Nuclear Research
Discovery independently by the Lawrence Berkeley Laboratory and the Joint Institute for Nuclear Research (1970)
Mass number [268]
Atomic number (Z) 105
Group group 5
Period period 7
Block d-block
Element category transition metal
Electron configuration [Rn] 5f14 6d3 7s2[3]
Electrons per shell
2, 8, 18, 32, 32, 11, 2
- Ds Darmstadtium 110
Symbol Ds
Naming after Darmstadt, Germany, where it was discovered
Discovery Gesellschaft für Schwerionenforschung (1994)
Mass number [281]
Atomic number (Z) 110
Group group 10
Period period 7
Block d-block
Element category unknown chemical properties, but probably a transition metal
Electron configuration [Rn] 5f14 6d8 7s2 (predicted)
Electrons per shell
2, 8, 18, 32, 32, 16, 2 (predicted)
- Dy Dysprosium 66
Symbol Dy
Discovery Lecoq de Boisbaudran (1886)
Appearance silvery white
Standard atomic weight Ar, std(Dy) 162.500(1)
Atomic number (Z) 66
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f10 6s2
Electrons per shell
2, 8, 18, 28, 8, 2
- Er Erbium 68
Symbol Er
Naming after Ytterby (Sweden), where it was mined
Discovery Carl Gustaf Mosander (1843)
Appearance silvery white
Standard atomic weight Ar, std(Er) 167.259(3)
Atomic number (Z) 68
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f12 6s2
Electrons per shell
2, 8, 18, 30, 8, 2
- Es Einsteinium 99
Symbol Es
Naming after Albert Einstein
Discovery Lawrence Berkeley National Laboratory (1952)
Appearance silvery; glows blue in the dark
Mass number [252]
Atomic number (Z) 99
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f11 7s2
Electrons per shell
2, 8, 18, 32, 29, 8, 2
- Eu Europium 63
Symbol Eu
Naming after Europe
Discovery and first isolation Eugène-Anatole Demarçay (1896, 1901)
Appearance silvery white, with a pale yellow tint; but rarely seen without oxide discoloration
Standard atomic weight Ar, std(Eu) 151.964(1)
Atomic number (Z) 63
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f7 6s2
Electrons per shell
2, 8, 18, 25, 8, 2
- F Fluorine 9
Symbol F
Naming after the mineral fluorite, itself named after Latin fluo (to flow, in smelting)
Discovery André-Marie Ampère (1810)
First isolation Henri Moissan (June 26, 1886)
Named by Humphry Davy
Appearance gas: very pale yellow
liquid: bright yellow
solid: alpha is opaque, beta is transparent
Standard atomic weight Ar, std(F) 18.998403163(6)
Atomic number (Z) 9
Group group 17 (halogens)
Period period 2
Block p-block
Element category reactive nonmetal
Electron configuration [He] 2s2 2p5[2]
Electrons per shell
2, 7
- Fl Flerovium 114
Symbol Fl
Naming after Flerov Laboratory of Nuclear Reactions (itself named after Georgy Flyorov)
Discovery Joint Institute for Nuclear Research (JINR) and Lawrence Livermore National Laboratory (LLNL) (1999)
Mass number [289] (unconfirmed: 290)
Atomic number (Z) 114
Group group 14 (carbon group)
Period period 7
Block p-block
Element category unknown chemical properties, but probably a post-transition metal
Electron configuration [Rn] 5f14 6d10 7s2 7p2 (predicted)[2]
Electrons per shell
2, 8, 18, 32, 32, 18, 4 (predicted)
- Fe Iron 26
Symbol Fe
Discovery before 5000 BC
Appearance lustrous metallic with a grayish tinge
Standard atomic weight Ar, std(Fe) 55.845(2)
Atomic number (Z) 26
Group group 8
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d6 4s2
Electrons per shell
2, 8, 14, 2
- Fm Fermium 100
Symbol Fm
Naming after Enrico Fermi
Discovery Lawrence Berkeley National Laboratory (1952)
Mass number [257]
Atomic number (Z) 100
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f12 7s2
Electrons per shell
2, 8, 18, 32, 30, 8, 2
- Fr Francium 87
Symbol Fr
Naming after France, homeland of the discoverer
Discovery and first isolation Marguerite Perey (1939)
Mass number [223]
Atomic number (Z) 87
Group group 1 (alkali metals)
Period period 7
Block s-block
Element category alkali metal
Electron configuration [Rn] 7s1
Electrons per shell
2, 8, 18, 32, 18, 8, 1
- Ga Gallium 31
Symbol Ga
Naming after Gallia (Latin for: France), homeland of the discoverer
Prediction Dmitri Mendeleev (1871)
Discovery and first isolation Lecoq de Boisbaudran (1875)
Appearance silvery blue
Standard atomic weight Ar, std(Ga) 69.723(1)
Atomic number (Z) 31
Group group 13 (boron group)
Period period 4
Block p-block
Element category post-transition metal
Electron configuration [Ar] 3d10 4s2 4p1
Electrons per shell
2, 8, 18, 3
- Gd Gadolinium 64
Symbol Gd
Naming after the mineral Gadolinite (itself named after Johan Gadolin)
Discovery Jean Charles Galissard de Marignac (1880)
First isolation Lecoq de Boisbaudran (1886)
Appearance silvery white
Standard atomic weight Ar, std(Gd) 157.25(3)
Atomic number (Z) 64
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f7 5d1 6s2
Electrons per shell
2, 8, 18, 25, 9, 2
- Ge Germanium 32
Symbol Ge
Naming after Germany, homeland of the discoverer
Prediction Dmitri Mendeleev (1869)
Discovery Clemens Winkler (1886)
Appearance grayish-white
Standard atomic weight Ar, std(Ge) 72.630(8)
Atomic number (Z) 32
Group group 14 (carbon group)
Period period 4
Block p-block
Element category metalloid
Electron configuration [Ar] 3d10 4s2 4p2
Electrons per shell
2, 8, 18, 4
- H Hydrogen 1
Symbol H
Discovery Henry Cavendish (1766)
Named by Antoine Lavoisier (1783)
Appearance colorless gas
Standard atomic weight Ar, std(H) [1.00784, 1.00811] conventional: 1.008
Atomic number (Z) 1
Group group 1
Period period 1
Block s-block
Element category reactive nonmetal
Electron configuration 1s1
Electrons per shell
1
- He Helium 2
Symbol He
Naming after Helios, Greek Titan of the Sun
Discovery Pierre Janssen, Norman Lockyer (1868)
First isolation William Ramsay, Per Teodor Cleve, Abraham Langlet (1895)
Appearance colorless gas, exhibiting a gray, cloudy glow (or reddish-orange if an especially high voltage is used) when placed in an electric field
Standard atomic weight Ar, std(He) 4.002602(2)
Atomic number (Z) 2
Group group 18 (noble gases)
Period period 1
Block s-block
Element category noble gas
Electron configuration 1s2
Electrons per shell
2
- Hf Hafnium 72
Symbol Hf
Naming after Hafnia. Latin for: Copenhagen, where it was discovered
Prediction Dmitri Mendeleev (1869)
Discovery and first isolation Dirk Coster and George de Hevesy (1922)
Appearance steel gray
Standard atomic weight Ar, std(Hf) 178.49(2)
Atomic number (Z) 72
Group group 4
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d2 6s2
Electrons per shell
2, 8, 18, 32, 10, 2
- Hg Mercury 80
Symbol Hg
Discovery Ancient Egyptians (before 1500 BCE)
Atomic number (Z) 80
Appearance silvery
Standard atomic weight Ar, std(Hg) 200.592(3)
Group group 12
Period period 6
Block d-block
Element category post-transition metal, alternatively considered a transition metal
Electron configuration [Xe] 4f14 5d10 6s2
Electrons per shell
2, 8, 18, 32, 18, 2
- Ho Holmium 67
Symbol Ho
Discovery Jacques-Louis Soret and Marc Delafontaine (1878)
Appearance silvery white
Standard atomic weight Ar, std(Ho) 164.930328(7)
Atomic number (Z) 67
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f11 6s2
Electrons per shell
2, 8, 18, 29, 8, 2
- Hs Hassium 108
Symbol Hs
Naming after Hassia, Latin for Hesse, Germany, where it was discovered
Discovery Gesellschaft für Schwerionenforschung (1984)
Mass number [269]
Atomic number (Z) 108
Group group 8
Period period 7
Block d-block
Element category transition metal
Electron configuration [Rn] 5f14 6d6 7s2[3]
Electrons per shell
2, 8, 18, 32, 32, 14, 2
- I Iodine 53
Symbol I
Discovery and first isolation Bernard Courtois (1811)
Appearance lustrous metallic gray, violet as a gas
Standard atomic weight Ar, std(I) 126.90447(3)
Atomic number (Z) 53
Group group 17 (halogens)
Period period 5
Block p-block
Element category reactive nonmetal
Electron configuration [Kr] 4d10 5s2 5p5
Electrons per shell
2, 8, 18, 18, 7
- In Indium 49
Symbol In
Discovery Ferdinand Reich and Hieronymous Theodor Richter (1863)
First isolation Hieronymous Theodor Richter (1864)
Appearance silvery lustrous gray
Standard atomic weight Ar, std(In) 114.818(1)
Atomic number (Z) 49
Group group 13 (boron group)
Period period 5
Block p-block
Element category post-transition metal
Electron configuration [Kr] 4d10 5s2 5p1
Electrons per shell
2, 8, 18, 18, 3
- Ir Iridium 77
Symbol Ir
Discovery and first isolation Smithson Tennant (1803)
Appearance silvery white
Standard atomic weight Ar, std(Ir) 192.217(2)
Atomic number (Z) 77
Group group 9
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d7 6s2
Electrons per shell
2, 8, 18, 32, 15, 2
- K Potassium 19
Symbol K
Discovery and first isolation Humphry Davy (1807)
Appearance silvery gray
Standard atomic weight Ar, std(K) 39.0983(1)
Atomic number (Z) 19
Group group 1 (alkali metals)
Period period 4
Block s-block
Element category alkali metal
Electron configuration [Ar] 4s1
Electrons per shell
2, 8, 8, 1
- Kr Krypton 36
Symbol Kr
Discovery and first isolation William Ramsay and Morris Travers (1898)
Appearance colorless gas, exhibiting a whitish glow in an electric field
Standard atomic weight Ar, std(Kr) 83.798(2)
Atomic number (Z) 36
Group group 18 (noble gases)
Period period 4
Block p-block
Element category noble gas
Electron configuration [Ar] 3d10 4s2 4p6
Electrons per shell
2, 8, 18, 8
- La Lanthanum 57
Symbol La
Discovery Carl Gustaf Mosander (1838)
Appearance silvery white
Standard atomic weight Ar, std(La) 138.90547(7)
Atomic number (Z) 57
Group group 3
Period period 6
Block d-block
Element category lanthanide, sometimes considered a transition metal
Electron configuration [Xe] 5d1 6s2
Electrons per shell
2, 8, 18, 18, 9, 2
- Lr Lawrencium 103
Symbol Lr
Naming after Ernest Lawrence
Discovery Lawrence Berkeley National Laboratory and Joint Institute for Nuclear Research (1961–1971)
Appearance silvery (predicted)
Mass number [266]
Atomic number (Z) 103
Group group n/a
Period period 7
Block f-block
Element category actinide, sometimes considered a transition metal
Electron configuration [Rn] 5f14 7s2 7p1
Electrons per shell
2, 8, 18, 32, 32, 8, 3
- Li Lithium 3
Symbol Li
Discovery Johan August Arfwedson (1817)
First isolation William Thomas Brande (1821)
Appearance silvery-white
Standard atomic weight Ar, std(Li) [6.938, 6.997] conventional: 6.94
Atomic number (Z) 3
Group group 1 (alkali metals)
Period period 2
Block s-block
Element category alkali metal
Electron configuration [He] 2s1
Electrons per shell
2, 1
- Lv Livermorium 116
Symbol Lv
Naming after Lawrence Livermore National Laboratory, itself named partly after Livermore, California
Discovery Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2000)
Mass number [293]
Atomic number (Z) 116
Group group 16 (chalcogens)
Period period 7
Block p-block
Element category unknown chemical properties, but probably a post-transition metal
Electron configuration [Rn] 5f14 6d10 7s2 7p4 (predicted)[1]
Electrons per shell
2, 8, 18, 32, 32, 18, 6 (predicted)
- Lu Lutetium 71
Symbol Lu
Naming after Lutetia, Latin for: Paris, in the Roman era
Discovery Carl Auer von Welsbach and Georges Urbain (1906)
First isolation Carl Auer von Welsbach (1906)
Named by Georges Urbain (1906)
Appearance silvery white
Standard atomic weight Ar, std(Lu) 174.9668(1)
Atomic number (Z) 71
Group group n/a
Period period 6
Block f-block
Element category lanthanide, sometimes considered a transition metal
Electron configuration [Xe] 4f14 5d1 6s2
Electrons per shell
2, 8, 18, 32, 9, 2
- Md Mendelevium 101
Symbol Md
Naming after Dmitri Mendeleev
Discovery Lawrence Berkeley National Laboratory (1955)
Mass number [258]
Atomic number (Z) 101
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f13 7s2
Electrons per shell
2, 8, 18, 32, 31, 8, 2
- Mg Magnesium 12
Symbol Mg
Naming after Magnesia, Greece
Discovery Joseph Black (1755)
First isolation Humphry Davy (1808)
Appearance shiny grey solid
Standard atomic weight Ar, std(Mg) [24.304, 24.307] conventional: 24.305
Atomic number (Z) 12
Group group 2 (alkaline earth metals)
Period period 3
Block s-block
Element category alkaline earth metal
Electron configuration [Ne] 3s2
Electrons per shell
2, 8, 2
- Mn Manganese 25
Symbol Mn
Discovery Carl Wilhelm Scheele (1774)
First isolation Johann Gottlieb Gahn (1774)
Appearance silvery metallic
Standard atomic weight Ar, std(Mn) 54.938043(2)
Atomic number (Z) 25
Group group 7
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d5 4s2
Electrons per shell
2, 8, 13, 2
- Mt Meitnerium 109
Symbol Mt
Naming after Lise Meitner
Discovery Gesellschaft für Schwerionenforschung (1982)
Mass number [278] (unconfirmed: 282)
Atomic number (Z) 109
Group group 9
Period period 7
Block d-block
Element category unknown chemical properties, but probably a transition metal[3][4]
Electron configuration [Rn] 5f14 6d7 7s2 (predicted)[3][5]
Electrons per shell
2, 8, 18, 32, 32, 15, 2 (predicted)
- Mo Molybdenum 42
Symbol Mo
Discovery Carl Wilhelm Scheele (1778)
First isolation Peter Jacob Hjelm (1781)
Appearance gray metallic
Standard atomic weight Ar, std(Mo) 95.95(1)
Atomic number (Z) 42
Group group 6
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d5 5s1
Electrons per shell
2, 8, 18, 13, 1
- Mc Moscovium 115
Symbol Mc
Naming After Moscow region
Discovery Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2003)
Mass number [290]
Atomic number (Z) 115
Group group 15 (pnictogens)
Period period 7
Block p-block
Element category unknown chemical properties, but probably a post-transition metal
Electron configuration [Rn] 5f14 6d10 7s2 7p3 (predicted)[1]
Electrons per shell
2, 8, 18, 32, 32, 18, 5 (predicted)
- N Nitrogen 7
Symbol N
Discovery Daniel Rutherford (1772)
Named by Jean-Antoine Chaptal (1790)
Appearance colorless gas, liquid or solid
Standard atomic weight Ar, std(N) [14.00643, 14.00728] conventional: 14.007
Atomic number (Z) 7
Group group 15 (pnictogens)
Period period 2
Block p-block
Element category reactive nonmetal
Electron configuration [He] 2s2 2p3
Electrons per shell
2, 5
- Na Sodium 11
Symbol Na
Discovery and first isolation Humphry Davy (1807)
Appearance silvery white metallic
Standard atomic weight Ar, std(Na) 22.98976928(2)
Atomic number (Z) 11
Group group 1 (alkali metals)
Period period 3
Block s-block
Element category alkali metal
Electron configuration [Ne] 3s1
Electrons per shell
2, 8, 1
- Nb Niobium 41
Symbol Nb
Naming after Niobe in Greek mythology, daughter of Tantalus (tantalum)
Discovery Charles Hatchett (1801)
First isolation Christian Wilhelm Blomstrand (1864)
Recognized as a distinct element by Heinrich Rose (1844)
Appearance gray metallic, bluish when oxidized
Standard atomic weight Ar, std(Nb) 92.90637(1)
Atomic number (Z) 41
Group group 5
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d4 5s1
Electrons per shell
2, 8, 18, 12, 1
- Nd Neodymium 60
Symbol Nd
Discovery Carl Auer von Welsbach (1885)
Appearance silvery white
Standard atomic weight Ar, std(Nd) 144.242(3)
Atomic number (Z) 60
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f4 6s2
Electrons per shell
2, 8, 18, 22, 8, 2
- Ni Nickel 28
Symbol Ni
Discovery and first isolation Axel Fredrik Cronstedt (1751)
Appearance lustrous, metallic, and silver with a gold tinge
Standard atomic weight Ar, std(Ni) 58.6934(4)
Atomic number (Z) 28
Group group 10
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d8 4s2 or [Ar] 3d9 4s1
Electrons per shell
2, 8, 16, 2 or 2, 8, 17, 1
- Nh Nihonium 113
Symbol Nh
Naming After Japan (Nihon in Japanese)
Discovery Riken (Japan, first undisputed claim 2004)
JINR (Russia) and Livermore (US, first announcement 2003)
Mass number [286]
Atomic number (Z) 113
Group group 13 (boron group)
Period period 7
Block p-block
Element category unknown chemical properties, but probably a post-transition metal
Electron configuration [Rn] 5f14 6d10 7s2 7p1 (predicted)[1]
Electrons per shell
2, 8, 18, 32, 32, 18, 3 (predicted)
- No Neon 10
Symbol No
Prediction William Ramsay (1897)
Discovery and first isolation William Ramsay & Morris Travers (1898)
Appearance colorless gas exhibiting an orange-red glow when placed in an electric field
Standard atomic weight Ar, std(Ne) 20.1797(6)
Atomic number (Z) 10
Group group 18 (noble gases)
Period period 2
Block p-block
Element category noble gas
Electron configuration [He] 2s2 2p6
Electrons per shell
2, 8
- No Nobelium 102
Symbol No
Naming after Alfred Nobel
Discovery Joint Institute for Nuclear Research (1966)
Mass number [259]
Atomic number (Z) 102
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f14 7s2
Electrons per shell
2, 8, 18, 32, 32, 8, 2
- Np Neptunium 93
Symbol Np
Naming after planet Neptune, itself named after Roman god of the sea Neptune
Discovery Edwin McMillan and Philip H. Abelson (1940)
Appearance silvery metallic
Mass number [237]
Atomic number (Z) 93
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f4 6d1 7s2
Electrons per shell
2, 8, 18, 32, 22, 9, 2
- Og Oganesson 118
Symbol Og
Naming after Yuri Oganessian
Prediction Hans Peter Jørgen Julius Thomsen (1895)
Discovery Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2002)
Mass number [294] (unconfirmed: 295)
Atomic number (Z) 118
Group group 18
Period period 7
Block p-block
Element category unknown chemical properties, but probably a noble gas
Electron configuration [Rn] 5f14 6d10 7s2 7p6 (predicted)[2][3]
Electrons per shell
2, 8, 18, 32, 32, 18, 8 (predicted)
- O Oxygen 8
Symbol O
Discovery Carl Wilhelm Scheele (1771)
Named by Antoine Lavoisier (1777)
Appearance gas: colorless
liquid and solid: pale blue
Standard atomic weight Ar, std(O) [15.99903, 15.99977] conventional: 15.999
Atomic number (Z) 8
Group group 16 (chalcogens)
Period period 2
Block p-block
Element category reactive nonmetal
Electron configuration [He] 2s2 2p4
Electrons per shell
2, 6
- Osmium 76
Symbol Os
Discovery and first isolation Smithson Tennant (1803)
Appearance silvery, blue cast
Standard atomic weight Ar, std(Os) 190.23(3)
Atomic number (Z) 76
Group group 8
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d6 6s2
Electrons per shell
2, 8, 18, 32, 14, 2
- P Phosphorus 15
Symbol P
Discovery Hennig Brand (1669)
Recognised as an element by Antoine Lavoisier (1777)
Appearance Colourless, waxy white, yellow, scarlet, red, violet, black
Standard atomic weight Ar, std(P) 30.973761998(5)
Atomic number (Z) 15
Group group 15 (pnictogens)
Period period 3
Block p-block
Element category reactive nonmetal
Electron configuration [Ne] 3s2 3p3
Electrons per shell
2, 8, 5
- Pa Protactinium 91
Symbol Pa
Prediction Dmitri Mendeleev (1869)
Discovery and first isolation Kasimir Fajans and Oswald Helmuth Göhring (1913)
Named by Otto Hahn and Lise Meitner (1917–8)
Appearance bright, silvery metallic luster
Standard atomic weight Ar, std(Pa) 231.03588(1)
Atomic number (Z) 91
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f2 6d1 7s2
Electrons per shell
2, 8, 18, 32, 20, 9, 2
- Pb Lead 82
Symbol Pb
Discovery in the Middle East (7000 BCE)
Appearance metallic gray
Standard atomic weight Ar, std(Pb) 207.2(1)
Atomic number (Z) 82
Group group 14 (carbon group)
Period period 6
Block p-block
Element category post-transition metal
Electron configuration [Xe] 4f14 5d10 6s2 6p2
Electrons per shell
2, 8, 18, 32, 18, 4
- Pd Palladium 46
Symbol Pd
Naming after asteroid Pallas, itself named after Pallas Athena
Discovery and first isolation William Hyde Wollaston (1802)
Appearance silvery white
Standard atomic weight Ar, std(Pd) 106.42(1)
Atomic number (Z) 46
Group group 10
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d10
Electrons per shell
2, 8, 18, 18
- Pm Promethium 61
Symbol Pm
Discovery Chien Shiung Wu, Emilio Segrè, Hans Bethe (1942)
First isolation Charles D. Coryell, Jacob A. Marinsky, Lawrence E. Glendenin (1945)
Named by Grace Mary Coryell (1945)
Appearance metallic
Mass number [145]
Atomic number (Z) 61
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f5 6s2
Electrons per shell
2, 8, 18, 23, 8, 2
- Po Polonium 84
Symbol Po
Naming after Polonia, Latin for Poland, homeland of Marie Curie
Discovery Pierre and Marie Curie (1898)
First isolation Willy Marckwald (1902)
Appearance silvery
Mass number [209]
Atomic number (Z) 84
Group group 16 (chalcogens)
Period period 6
Block p-block
Element category post-transition metal, but this status is disputed
Electron configuration [Xe] 4f14 5d10 6s2 6p4
Electrons per shell
2, 8, 18, 32, 18, 6
- Pr Praseodymium 59
Symbol Pr
Discovery Carl Auer von Welsbach (1885)
Appearance grayish white
Standard atomic weight Ar, std(Pr) 140.90766(1)
Atomic number (Z) 59
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f3 6s2
Electrons per shell
2, 8, 18, 21, 8, 2
- Pt Platinum 78
Symbol Pt
Discovery Antonio de Ulloa (1735)
Appearance silvery white
Standard atomic weight Ar, std(Pt) 195.084(9)
Atomic number (Z) 78
Group group 10
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d9 6s1
Electrons per shell
2, 8, 18, 32, 17, 1
- Pu Plutonium 94
Symbol Pu
Naming after dwarf planet Pluto, itself named after classical god of the underworld Pluto
Discovery Glenn T. Seaborg, Arthur Wahl, Joseph W. Kennedy, Edwin McMillan (1940–1)
Appearance silvery white, tarnishing to dark gray in air
Mass number [244]
Atomic number (Z) 94
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f6 7s2
Electrons per shell
2, 8, 18, 32, 24, 8, 2
- Ra Radium 88
Symbol Ra
Discovery Pierre and Marie Curie (1898)
First isolation Marie Curie (1910)
Appearance silvery white metallic
Mass number [226]
Atomic number (Z) 88
Group group 2 (alkaline earth metals)
Period period 7
Block s-block
Element category alkaline earth metal
Electron configuration [Rn] 7s2
Electrons per shell
2, 8, 18, 32, 18, 8, 2
- Rb Rubidium 37
Symbol Rb
Discovery Robert Bunsen and Gustav Kirchhoff (1861)
First isolation George de Hevesy
Appearance grey white
Standard atomic weight Ar, std(Rb) 85.4678(3)
Atomic number (Z) 37
Group group 1 (alkali metals)
Period period 5
Block s-block
Element category alkali metal
Electron configuration [Kr] 5s1
Electrons per shell
2, 8, 18, 8, 1
- Re Rhenium 75
Symbol Re
Naming after the river Rhine (German: Rhein)
Discovery Masataka Ogawa (1908)
First isolation Masataka Ogawa (1919)
Named by Walter Noddack, Ida Noddack, Otto Berg (1925)
Appearance silvery-grayish
Standard atomic weight Ar, std(Re) 186.207(1)
Atomic number (Z) 75
Group group 7
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d5 6s2
Electrons per shell
2, 8, 18, 32, 13, 2
- Rf Rutherfordium 104
Symbol Rf
Naming after Ernest Rutherford
Discovery Joint Institute for Nuclear Research and Lawrence Berkeley National Laboratory (1964, 1969)
Mass number [267]
Atomic number (Z) 104
Group group 4
Period period 7
Block d-block
Element category transition metal
Electron configuration [Rn] 5f14 6d2 7s2[1][2]
Electrons per shell
2, 8, 18, 32, 32, 10, 2
- Rg Roentgenium 111
Symbol Rg
Naming after Wilhelm Röntgen
Discovery Gesellschaft für Schwerionenforschung (1994)
Appearance silvery (predicted)
Mass number [282] (unconfirmed: 286)
Atomic number (Z) 111
Group group 11
Period period 7
Block d-block
Element category unknown chemical properties, but probably a transition metal
Electron configuration [Rn] 5f14 6d9 7s2 (predicted)[1][2]
Electrons per shell
2, 8, 18, 32, 32, 17, 2 (predicted)
- Rh Rhodium 45
Symbol Rh
Discovery and first isolation William Hyde Wollaston (1804)
Appearance silvery white metallic
Standard atomic weight Ar, std(Rh) 102.90549(2)
Atomic number (Z) 45
Group group 9
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d8 5s1
Electrons per shell
2, 8, 18, 16, 1
- Rn Radon 86
Symbol Rn
Discovery Ernest Rutherford and Robert B. Owens (1899)
First isolation William Ramsay and Robert Whytlaw-Gray (1910)
Appearance colorless gas
Mass number [222]
Atomic number (Z) 86
Group group 18 (noble gases)
Period period 6
Block p-block
Element category noble gas
Electron configuration [Xe] 4f14 5d10 6s2 6p6
Electrons per shell
2, 8, 18, 32, 18, 8
- Ru Ruthenium 44
Symbol Ru
Naming after Ruthenia (Latin for: medieval Kyivska Rus' region)
Discovery and first isolation Karl Ernst Claus (1844)
Appearance silvery white metallic
Standard atomic weight Ar, std(Ru) 101.07(2)
Atomic number (Z) 44
Group group 8
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d7 5s1
Electrons per shell
2, 8, 18, 15, 1
- S Sulfur 16
Symbol S
Discovery Chinese (before 2000 BCE)
Recognized as an element by Antoine Lavoisier (1777)
Appearance lemon yellow sintered microcrystals
Standard atomic weight Ar, std(S) [32.059, 32.076] conventional: 32.06
Atomic number (Z) 16
Group group 16 (chalcogens)
Period period 3
Block p-block
Element category reactive nonmetal
Electron configuration [Ne] 3s2 3p4
Electrons per shell
2, 8, 6
- Sb Antimony 51
Symbol Sb
Discovery Arabic alchemists (before AD 815)
Appearance silvery lustrous gray
Standard atomic weight Ar, std(Sb) 121.760(1)
Atomic number (Z) 51
Group group 15 (pnictogens)
Period period 5
Block p-block
Element category metalloid
Electron configuration [Kr] 4d10 5s2 5p3
Electrons per shell
2, 8, 18, 18, 5
- Sc Scandium 21
Symbol Sc
Naming after Scandinavia
Prediction Dmitri Mendeleev (1871)
Discovery and first isolation Lars Fredrik Ni
Appearance silvery white
Standard atomic weight Ar, std(Sc) 44.955908(5)
Atomic number (Z) 21
Group group 3
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d1 4s2
Electrons per shell
2, 8, 9, 2
- Se Selenium 34
Symbol Se
Naming after Selene, Greek goddess of the moon
Discovery and first isolation Jöns Jakob Berzelius and Johann Gottlieb Gahn (1817)
Appearance black, red, and gray allotropes
Standard atomic weight Ar, std(Se) 78.971(8)
Atomic number (Z) 34
Group group 16 (chalcogens)
Period period 4
Block p-block
Element category reactive nonmetal, sometimes considered a metalloid
Electron configuration [Ar] 3d10 4s2 4p4
Electrons per shell
2, 8, 18, 6
- Sg Seaborgium 106
Symbol Sg
Naming after Glenn T. Seaborg
Discovery Lawrence Berkeley National Laboratory (1974)
Mass number [269]
Atomic number (Z) 106
Group group 6
Period period 7
Block d-block
Element category transition metal
Electron configuration [Rn] 5f14 6d4 7s2[1]
Electrons per shell
2, 8, 18, 32, 32, 12, 2
- Si Silicon 14
Symbol Si
Naming after Latin 'silex' or 'silicis', meaning flint
Prediction Antoine Lavoisier (1787)
Discovery and first isolation Jöns Jacob Berzelius (1823)
Named by Thomas Thomson (1817)
Appearance crystalline, reflective with bluish-tinged faces
Standard atomic weight Ar, std(Si) [28.084, 28.086] conventional: 28.085
Atomic number (Z) 14
Group group 14 (carbon group)
Period period 3
Block p-block
Element category metalloid
Electron configuration [Ne] 3s2 3p2
Electrons per shell
2, 8, 4
- Sm Samarium 62
Symbol Sm
Naming after the mineral samarskite (itself named after Vassili Samarsky-Bykhovets)
Discovery and first isolation Lecoq de Boisbaudran (1879)
Appearance silvery white
Standard atomic weight Ar, std(Sm) 150.36(2)
Atomic number (Z) 62
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f6 6s2
Electrons per shell
2, 8, 18, 24, 8, 2
- Sn Tin 50
Symbol Sn
Discovery around 3500 BC
Appearance silvery-white (beta, β) or gray (alpha, α)
Standard atomic weight Ar, std(Sn) 118.710(7)
Atomic number (Z) 50
Group group 14 (carbon group)
Period period 5
Block p-block
Element category post-transition metal
Electron configuration [Kr] 4d10 5s2 5p2
Electrons per shell
2, 8, 18, 18, 4
- Sr Strontium 38
Symbol Sr
Naming after the mineral strontianite, itself named after Strontian, Scotland
Discovery William Cruickshank (1787)
First isolation Humphry Davy (1808)
Appearance silvery white metallic; with a pale yellow tint
Standard atomic weight Ar, std(Sr) 87.62(1)
Atomic number (Z) 38
Group group 2 (alkaline earth metals)
Period period 5
Block s-block
Element category alkaline earth metal
Electron configuration [Kr] 5s2
Electrons per shell
2, 8, 18, 8, 2
- Ta Tantalum 73
Symbol Ta
Discovery Anders Gustaf Ekeberg (1802)
Recognized as a distinct element by Heinrich Rose (1844)
Appearance gray blue
Standard atomic weight Ar, std(Ta) 180.94788(2)
Atomic number (Z) 73
Group group 5
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d3 6s2
Electrons per shell
2, 8, 18, 32, 11, 2
- Tb Terbium 65
Symbol Tb
Naming after Ytterby (Sweden), where it was mined
Discovery and first isolation Carl Gustaf Mosander (1843)
Appearance silvery white
Standard atomic weight Ar, std(Tb) 158.925354(8)
Atomic number (Z) 65
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f9 6s2
Electrons per shell
2, 8, 18, 27, 8, 2
- Tc Technetium 43
Symbol Tc
Prediction Dmitri Mendeleev (1871)
Discovery and first isolation Emilio Segrè and Carlo Perrier (1937)
Appearance shiny gray metal
Mass number [97]
Atomic number (Z) 43
Group group 7
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d5 5s2
Electrons per shell
2, 8, 18, 13, 2
- Te Tellurium 52
Symbol Te
Naming after Roman Tellus, deity of the Earth
Discovery Franz-Joseph Müller von Reichenstein (1782)
First isolation Martin Heinrich Klaproth
Appearance silvery lustrous gray (crystalline),
brown-black powder (amorphous)
Standard atomic weight Ar, std(Te) 127.60(3)
Atomic number (Z) 52
Group group 16 (chalcogens)
Period period 5
Block p-block
Element category metalloid
Electron configuration [Kr] 4d10 5s2 5p4
Electrons per shell
2, 8, 18, 18, 6
- Th Thorium 90
Symbol Th
Naming after Thor, the Norse god of thunder
Discovery Jöns Jakob Berzelius (1829)
Appearance silvery, often with black tarnish
Standard atomic weight Ar, std(Th) 232.0377(4)
Atomic number (Z) 90
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 6d2 7s2
Electrons per shell
2, 8, 18, 32, 18, 10, 2
- Ti Titanium 22
Symbol Ti
Discovery William Gregor (1791)
First isolation Jöns Jakob Berzelius (1825)
Named by Martin Heinrich Klaproth (1795)
Appearance silvery grey-white metallic
Standard atomic weight Ar, std(Ti) 47.867(1)
Atomic number (Z) 22
Group group 4
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d2 4s2
Electrons per shell
2, 8, 10, 2
- Tl Thallium 81
Symbol Tl
Naming after Greek thallos, green shoot or twig
Discovery William Crookes (1861)
First isolation Claude-Auguste Lamy (1862)
Appearance silvery white
Standard atomic weight Ar, std(Tl) [204.382, 204.385] conventional: 204.38
Atomic number (Z) 81
Group group 13 (boron group)
Period period 6
Block p-block
Element category post-transition metal
Electron configuration [Xe] 4f14 5d10 6s2 6p1
Electrons per shell
2, 8, 18, 32, 18, 3
- Tm Thulium 69
Symbol Tm
Naming after Thule, a mythical region in Scandinavia
Discovery and first isolation Per Teodor Cleve (1879)
Appearance silvery gray
Standard atomic weight Ar, std(Tm) 168.934218(6)
Atomic number (Z) 69
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f13 6s2
Electrons per shell
2, 8, 18, 31, 8, 2
- Ts Tennessine 117
Symbol Ts
Naming after Tennessee region
Discovery Joint Institute for Nuclear Research, Lawrence Livermore National Laboratory, Vanderbilt University and Oak Ridge National Laboratory (2009)
Appearance semimetallic (predicted)
Mass number [294]
Atomic number (Z) 117
Group group 17
Period period 7
Block p-block
Element category unknown chemical properties, but probably a post-transition metal[3][4]
Electron configuration [Rn] 5f14 6d10 7s2 7p5 (predicted)[5]
Electrons per shell
2, 8, 18, 32, 32, 18, 7 (predicted)
- U Uranium 92
Symbol U
Naming after planet Uranus, itself named after Greek god of the sky Uranus
Discovery Martin Heinrich Klaproth (1789)
First isolation Eugène-Melchior Péligot (1841)
Appearance silvery gray metallic; corrodes to a spalling black oxide coat in air
Standard atomic weight Ar, std(U) 238.02891(3)
Atomic number (Z) 92
Group group n/a
Period period 7
Block f-block
Element category actinide
Electron configuration [Rn] 5f3 6d1 7s2
Electrons per shell
2, 8, 18, 32, 21, 9, 2
- V Vanadium 23
Symbol V
Discovery Andrés Manuel del Río (1801)
First isolation Nils Gabriel Sefström (1830)
Named by Nils Gabriel Sefström (1830)
Appearance blue-silver-grey metal
Standard atomic weight Ar, std(V) 50.9415(1)
Atomic number (Z) 23
Group group 5
Period period 4
Block d-block
Element category transition metal
Electron configuration [Ar] 3d3 4s2
Electrons per shell
2, 8, 11, 2
- W Tungsten 74
Symbol W
Discovery Carl Wilhelm Scheele (1781)
First isolation Juan José Elhuyar and Fausto Elhuyar (1783)
Named by Torbern Bergman (1781)
Appearance grayish white, lustrous
Standard atomic weight Ar, std(W) 183.84(1)
Atomic number (Z) 74
Group group 6
Period period 6
Block d-block
Element category transition metal
Electron configuration [Xe] 4f14 5d4 6s2[2]
Electrons per shell
2, 8, 18, 32, 12, 2
- Xe Xenon 54
Symbol Xe
Discovery and first isolation William Ramsay and Morris Travers (1898)
Appearance colorless gas, exhibiting a blue glow when placed in an electric field
Standard atomic weight Ar, std(Xe) 131.293(6)
Atomic number (Z) 54
Group group 18 (noble gases)
Period period 5
Block p-block
Element category noble gas
Electron configuration [Kr] 4d10 5s2 5p6
Electrons per shell
2, 8, 18, 18, 8
- Y Yttrium 39
Symbol Y
Naming after Ytterby (Sweden) and its mineral ytterbite (gadolinite)
Discovery Johan Gadolin (1794)
First isolation Heinrich Rose (1843)
Appearance silvery white
Standard atomic weight Ar, std(Y) 88.90584(1)
Atomic number (Z) 39
Group group 3
Period period 5
Block d-block
Element category Transition metal
Electron configuration [Kr] 4d1 5s2
Electrons per shell
2, 8, 18, 9, 2
- Yb Ytterbium 70
Symbol Yb
Naming after Ytterby (Sweden), where it was mined
Discovery Jean Charles Galissard de Marignac (1878)
First isolation Carl Auer von Welsbach (1906)
Appearance silvery white; with a pale yellow tint
Standard atomic weight Ar, std(Yb) 173.045(10)
Atomic number (Z) 70
Group group n/a
Period period 6
Block f-block
Element category lanthanide
Electron configuration [Xe] 4f14 6s2
Electrons per shell
2, 8, 18, 32, 8, 2
- Zn Zinc 30
Symbol Zn
Discovery Indian metallurgists (before 1000 BCE)
First isolation Andreas Sigismund Marggraf (1746)
Recognized as a unique metal by Rasaratna Samuccaya (800)
Appearance silver-gray
Standard atomic weight Ar, std(Zn) 65.38(2)
Atomic number (Z) 30
Group group 12
Period period 4
Block d-block
Element category post-transition metal, alternatively considered a transition metal
Electron configuration [Ar] 3d10 4s2
Electrons per shell
2, 8, 18, 2
- Zr Zirconium 40
Symbol Zr
Naming after zircon, zargun زرگون meaning "gold-colored".
Discovery Martin Heinrich Klaproth (1789)
First isolation Jöns Jakob Berzelius (1824)
Appearance silvery white
Standard atomic weight Ar, std(Zr) 91.224(2)
Atomic number (Z) 40
Group group 4
Period period 5
Block d-block
Element category transition metal
Electron configuration [Kr] 4d2 5s2
Electrons per shell
2, 8, 18, 10, 2
Periodic table of the chemical elements
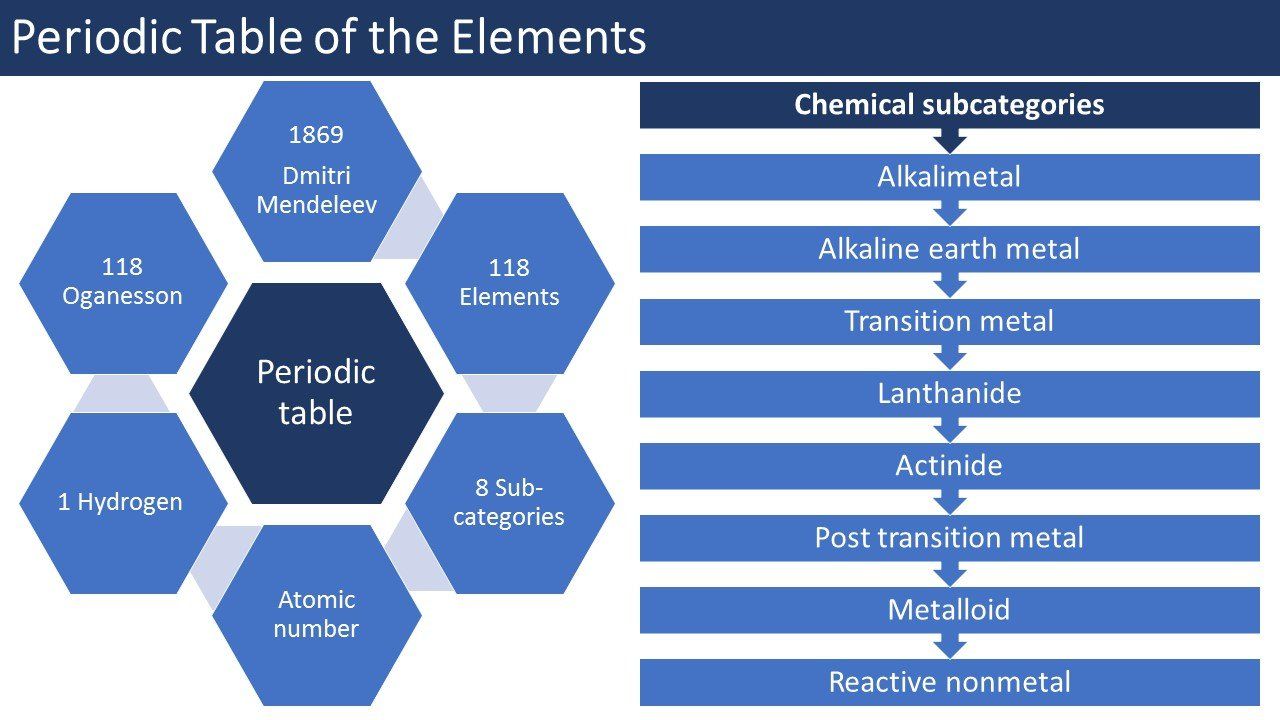
In the periodic table of elements tool we first show the above table with the chemical elements and the selection tool, after the table is the introduction text which is followed by a cool infographic. The list of elements is made for everybody ranging from laboratory students, chemistry students, chemistry teachers, chemists until persons that just have an interest in a certain chemical element. If you click on an element in a row then it will expand and more information about the specific element will be shown. The list contains the 118 chemical elements, starting with the element Actinium and the list ends with the element Zirconium. The following information will be shown about the specific chemical element.
For quick reference go to;
Atomic number: What is the atomic number definition? The atomic number of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element, so every element that exists has a unique number. The proton number or atomic number symbol is "Z".
Group: What are groups on the periodic table? The periodic table groups, which is also known as a family in chemistry, is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table. In the periodic table the f-block columns, between groups 3 and 4, are not numbered.
Period: What is the period on the periodic table? The period in the periodic table is a row of chemical elements on the periodic table. The elements in a row have the same number of electron shells.
Block: What are the blocks on the periodic table? The block of the periodic table of elements is a set of chemical elements that have differentiating electrons predominately in the same type of atomic orbital.
Elemental category: What are the elemental categories on the periodic table? The elemental category consists out of the chemical sub categories; Reactive nonmetal, alkali metal, alkaline earth metal, transition metal, lanthanide, actinide, post transition metal, metalloid, reactive none metal and noble gas.
Electron configuration: What are the electron configurations of a element? The electron configuration of the chemical element is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
Electrons per shell: What does the amount of electrons per shell mean? The electrons per shell does describe the amount of electrons that are present in a shell.